When correctly executed, the randomized managed trial is without doubt one of the greatest autos for assessing the effectiveness of a number of interventions. However, quite a few challenges might emerge within the areas of examine startup, recruitment, knowledge high quality, price, and reporting of outcomes.
The use of well-run coordinating facilities may assist forestall these points, however little or no exists within the literature describing their creation or the guiding rules behind their inception.
The Center for Clinical Trials & Data Coordination (CCDC) was established in 2015 by institutional funds with the intent of 1) offering related experience in clinical trial design, conduct, coordination, and evaluation; 2) advancing the careers of clinical investigators and CCDC-affiliated college; and 3) acquiring giant knowledge coordinating heart (DCC) grants.
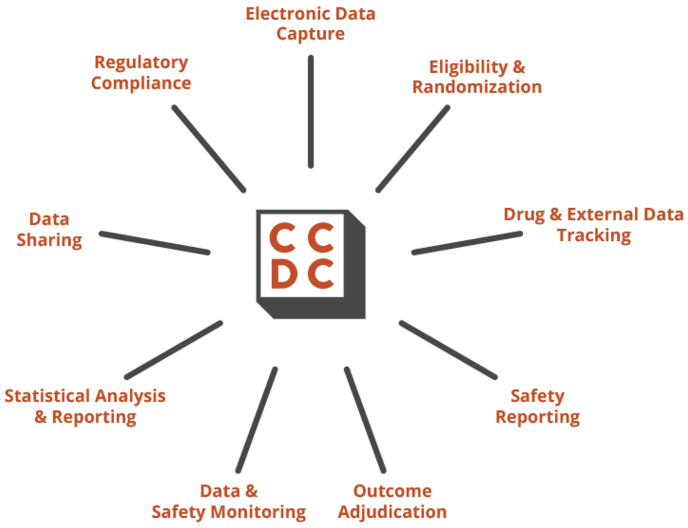
We describe the organizational construction of the CCDC in addition to the homegrown clinical trial administration system integrating 9 essential parts: digital knowledge seize, eligibility and randomization, drug and exterior knowledge monitoring, security reporting, final result adjudication, knowledge and security monitoring, statistical evaluation and reporting, knowledge sharing, and regulatory compliance. Lastly, we share quite a few classes that may be taken from our expertise.
Specifically, we concentrate on 1) funding for DCCs, 2) the significance of DCCs to clinical researchers, 3) the experience of DCC personnel, and 4) regularly striving to enhance. In conclusion, the CCDC strives to present high-quality assist for the design, conduct, coordination, and analyses of clinical trials, and we hope this paper will function a blueprint for future clinical trialists concerned in DCCs.
Twitter Activity Is Associated with a Higher Research Citation Index for Academic Thoracic Surgeons.
Academic surgeons are inspired to promote their work on social media. We hypothesized that thoracic surgeons who’re lively on Twitter have the next research quotation index (h-index) than their counterparts who will not be.Thoracic surgeons on CTSNet.org in Canada and the United States have been queried for profiles with an h-index on Google Scholar (GS) and/or Research Gate (RG) in July 2018.
Surgeons have been categorized by whether or not they possessed a Twitter account (T+) or not (T-), and h-index values have been in contrast. Within the T+ cohort, a multivariate regression mannequin was used to determine impartial predictors of elevated h-index amongst variables associated to Twitter exercise.RESULTSOf 3,741 surgeons queried, 19.3% (722) had a identified h-index.
The imply (SD) h-index for the complete cohort was 14.54 (15.73). The median (vary) h-index was 10 (0-121), and the 75th percentile h-index was 20. T+ surgeons had a median (vary) h-index of 10 (0-66), and T- surgeons had a median (vary) h-index of 10 (0-72, p=0.25). The 75th percentile h-index for T+ surgeons was 23, in contrast to 20 for T- surgeons (p=0.24). For T+ surgeons, the regression mannequin recognized the variety of followers (p=0.029), the variety of individuals adopted (p=0.048), and the frequency of tweeting (p=0.046) as impartial predictors of a better h-index.The median h-index for an academic thoracic surgeon in Canada and the United States is 10. Surgeons who have interaction in Twitter exercise are extra seemingly to have their research cited by others.
